AAV Vector Technologies for Gene Therapy
AAV Vector Technologies for Gene Therapy
AAV vectors are used in multiple gene therapy programs all over the world. About 30 percent of clinical gene therapy trials worldwide use this vector platform [1].
SIRION Biotech has been supporting pharmaceutical and biotech organizations for years enabling targeted gene therapy by providing our knowledge and IP in AAV capsid engineering and vector optimization to enhance manufacturability, target specificity and gene delivery ̶̶̶̶ crucial prerequisites to reduce vector dose and mitigate off-target safety issues.
Our holistic approach focusses on both AAV capsids and the transgene payload, creating new intellectual property (IP) tailored specifically to our partner’s needs.
In close collaboration with your experts and our network of alliance and academic partners, we decide on and apply the most suitable tools from our method toolbox for each individual project. These AAV-based technologies include:
Directed Evolution of AAV Capsids
AAV capsid directed evolution projects leverage our dynamic alliance network to tailor to the individual setup, methods, and technology needs of our clients. To optimize AAV tropism, we use a directed evolution approach via capsid randomization. This technology is made available through the partnership and license between SIRION Biotech and Prof. Dr. Dirk Grimm of Heidelberg University in Germany, a pioneer in AAV capsid evolution.
SIRION Biotech’s long-standing experience in AAV vector manufacturing at our upstream and downstream processing facility in Graefelfing is a key source for the required quality of capsids for these collaboration projects.
AAV directed capsid evolution entails:
-
Iterative in vivo screenings of recombinant AAV (rAAV) libraries harboring randomized peptide insertion libraries or AAV shuffled variants or combinations thereof
-
In vivo biodistribution analysis of enriched barcoded AAVs.
-
AAV lead candidates are identified by bioinformatic analyses of the data collections obtained from state-of-the-art next generation sequencing and PCR techniques from transduced tissue.
In addition to AAV capsid evolution, Nanobodies (see below) can be utilized as a direct targeting approach to further optimize AAV capsids.
Licensing Information
If you are interested in this technology, please get in touch with our Business Development and Licensing team at: licensing@sirion-biotech.com or use our contact form.
Building blocks of our directed evolution projects
Together with our clients, partners, and scientific network we are exploring new avenues regarding vector design, administration, preclinical in vivo models, and data analyses.
Our new site in Heidelberg
AAV evolution and vector development projects are conducted in our new Heidelberg laboratories, where our team of AAV specialists work closely with Prof. Grimm's research group to stay at the forefront of AAV capsid development.

Nanobody Targeting – Rational Capsid Design
Highly specific nanobodies are a novel rational design strategy that allow direct targeting of a surface protein of interest. This technology can be implemented separately or in combination with directed evolution (internal link) of AAV capsids to further enhance cell or tissue tropism and minimize off-target effects.
Nanobodies are Lama-derived heavy-chain antibody (VHH) fragments that combine small size (18 kDa) with high target specificity. Our scientific collaborator has proven the potential of nanobody-mediated targeting of engineered AAV capsids towards selected surface molecules of target cells. In the proof-of-concept study target-specific nanobodies were successfully fused to the VP1 capsid protein of AAV vectors to direct these engineered capsids to proteins of interest on the cell surface of target cells [1].
Including nanobody technology into future R&D projects will further advance AAV-mediated gene therapy by engineering safe, efficient, specific and potent prospective AAV vector candidates for clinical applications.
Licensing Information
If you are interested in this technology, please get in touch with our Business Development and Licensing team at: licensing@sirion-biotech.com or use our contact form.
References:
[1] Eichhoff et al. 2019
Therapeutic Expression Cassette Engineering
/Design%20and%20development%20of%20therapeutic%20expression%20cassettes%20Table.png?width=1732&height=962&name=Design%20and%20development%20of%20therapeutic%20expression%20cassettes%20Table.png)
The expression cassette is one of three key elements in rAAV vector design, and therefore crucial to improving the proportion of AAV gene therapy trials that meet their primary efficacy and safety endpoints. SIRION Biotech’s extensive expertise helps CGT developers engineer therapeutic expression cassettes with safe and efficacious levels of on-target expression while minimizing off-target effects. This requires sophisticated cassette design and selection based on the latest scientific and regulatory insights. Promoters, regulatory, and posttranscriptional elements are some of the many elements that can be optimized during transgene expression cassette design providing virtually endless expression cassette design possibilities.
To conquer this complexity, SIRION uses the DESIGN-BUILD-TEST-LEARN framework.
-
DESIGN – in-silico design of a vector candidate library
-
BUILD – manufacturing of plasmid and viral vector candidates
-
TEST – determining a testing strategy with appropriate read outs to identify the most favorable vector design
-
LEARN – Generate data on lead vector performance and feedback to a following development cycle
Our AAV specialists provide intensive analysis of transgene cassettes and can either support CGT developers in optimizing the design for their targeted application or develop a purpose tailored cassette de novo. Variables include, but are not limited to:
-
Promoter (constitutive, tissue- or cell specific)
-
Promoter proximal introns
-
regulatory RNAs
-
Therapeutic transgene identification, including shRNA selection using RNAiONE and base editing (links)
-
Transgene optimization, including tissue specific codon optimization
-
Posttranscriptional elements
-
Poly adenylation signals
-
Expression cassette size optimization
If you are interested in this technology, please get in touch with us via our contact form.
High Throughput Lead Vector Identification
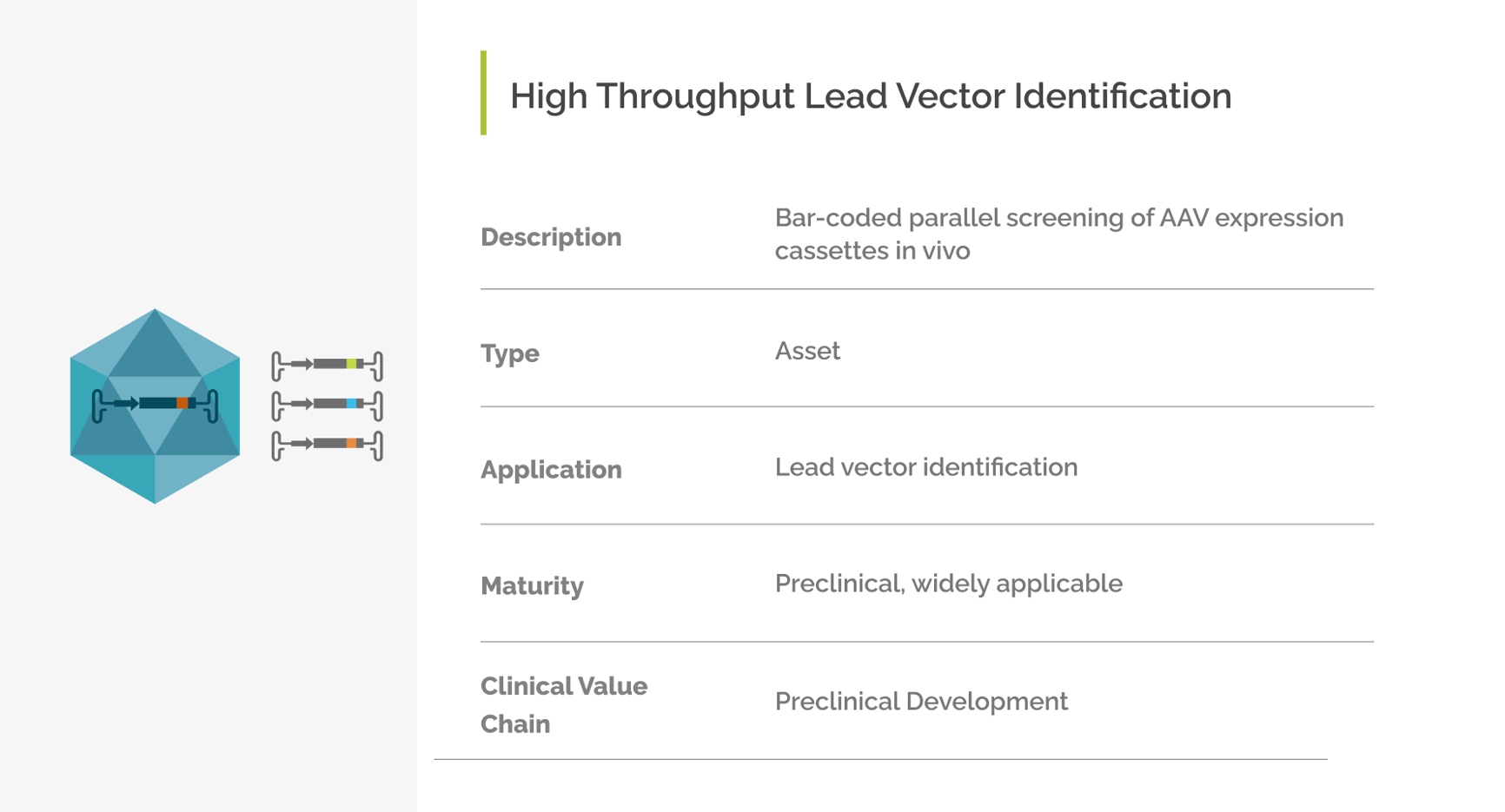
The barcoded AAV screening method developed by Prof. Dr. Dirk Grimm allows for parallel screening of candidate AAV vectors including expression cassettes in vivo. Bioinformatic analysis of the barcoded AAV library facilitates identification of one lead AAV vector with superior cell and/or tissue tropism and transgene expression levels in the target tissue or cell.
Licensing Information
If you are interested in this technology, please get in touch with our Business Development and Licensing team at: licensing@sirion-biotech.com or use our contact form.
Manufacturing Technologies
Optimized Manufacturing
SIRION Biotech’s ISO-certified manufacturing processes are optimized for minimal process- and product related impurities. For pre-clinical and clinical projects, USP and DSP can be adapted to meet client-specified quality attributes. To meet these quality attributes, we can perform both custom manufacturing plasmid development in research collaborations and process development of each step depicted below.
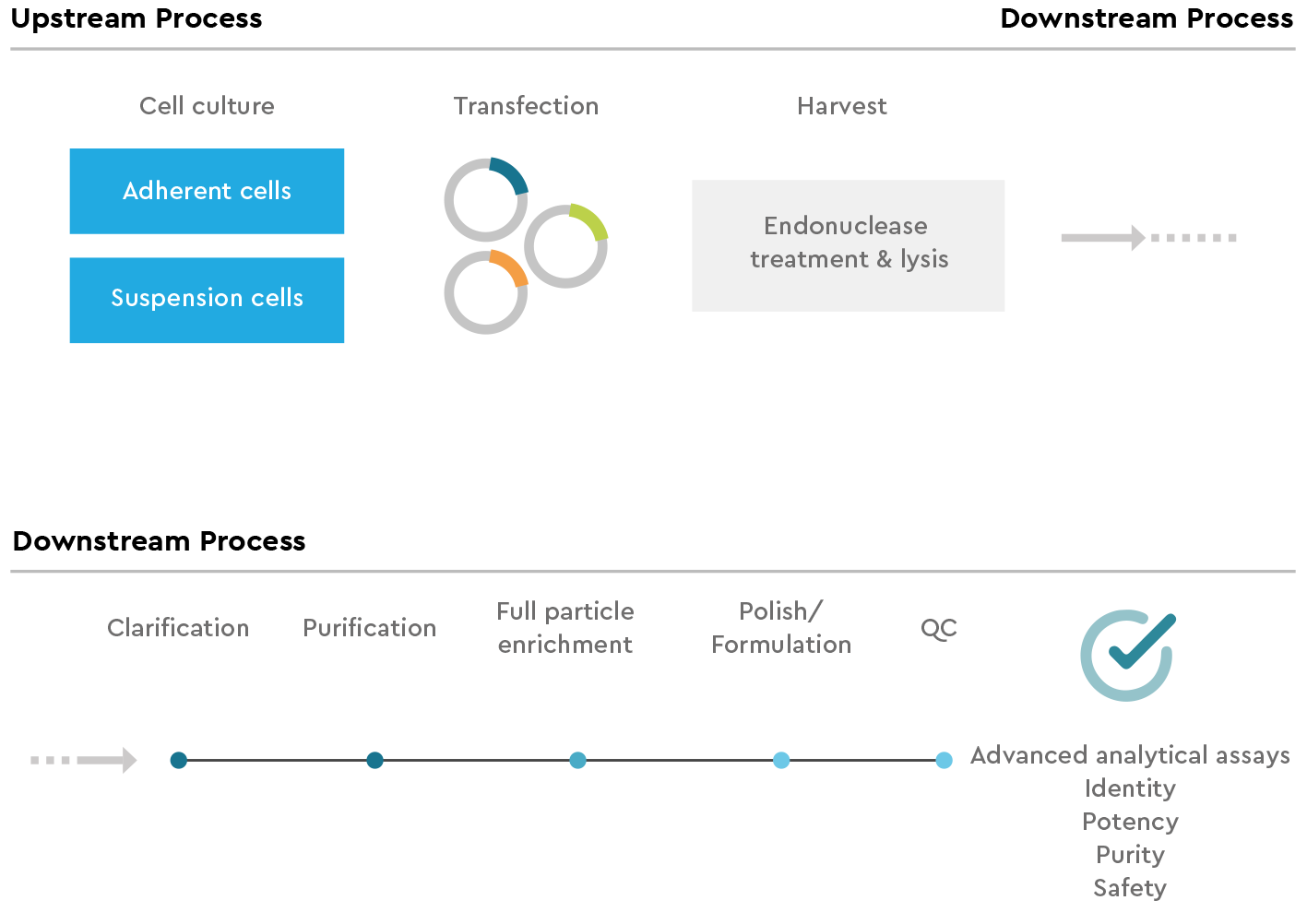
We offer a broad range of extended QCs to monitor and document purity, potency, safety and identity of the AAV vector plasmid backbone, therapeutic transgene cassette, and manufactured AAV vectors. The manufacturing procedures, QCs and documentation is specific to the project and tailored to the exact requirements of each CGT developer.
For GMP manufacturing, we work closely with a select group of CMOs to ensure speedy and efficient transfer of developed processes and analytics. For more information about the advantages SIRION Biotech can bring to accelerate project transfer, please visit our GMP Alliances page.
We are constantly updating and expanding our capabilities according to the latest scientific insights and are also excited to develop new assets to improve manufacturing cost, efficiency, safety, and productivity in collaboration with our CGT clients. If you are interested in any of these technologies, please get in touch with us via our contact form.
Plasmid backbones optimized for clinical use

SIRION developed AAV plasmid backbones optimized for GMP manufacturing and clinical use. The development of these backbones was guided by three goals:
- Minimal product-related impurities for enhanced vector safety
- Compliance with evolving regulatory guidelines
- High plasmid and AAV vector yields combined with reduced plasmid manufacturing cost
Our optimized plasmid backbone (COB) version 1.0 is currently used in GMP manufacturing for clinical trials.
If you are interested in our AAV vector backbones optimized for clinical use, please get in touch with us via our contact form.
