GMP Alliances
GMP Alliances
GMP Alliances ensures smooth process transfer from preclinical to clinical manufacturing.
Support for GMP Manufacturing
Successful cell and gene therapy and vaccine development requires effective cost and timeline control at all stages. SIRION supports and serves clients from early R&D through preclinical viral vector production and to clinical stage manufacturing.
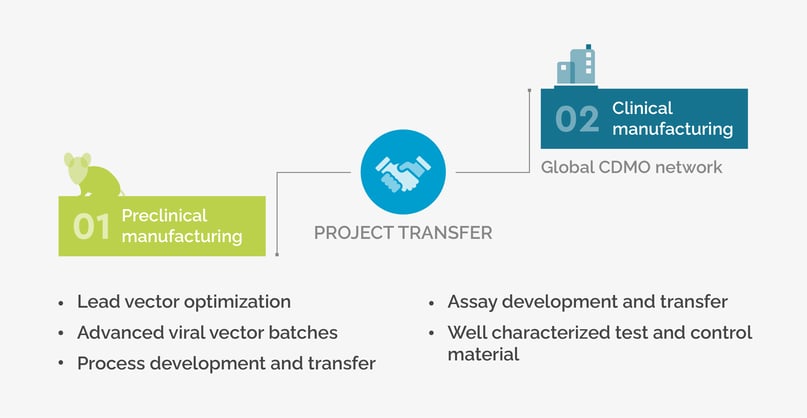
SIRION contributes to CGT success from vector development through to clinical manufacturing
Global CDMO Network
During the early R&D and preclinical project stages at SIRION, relevant individual project aspects are considered from the start to ensure a seamless clinical project transfer. SIRION has developed a global CDMO network over the last 5 years, and can recommend and introduce CDMOs worldwide that best suit customer location and individual project requirements.
SIRON supports you in bringing viral vector drug products to market as quickly and cost effectively as possible
Crucial time savings through smooth project transfer
Moving projects from the preclinical phase to a GMP manufacturing facility can require set up of entirely new processes and analytics. SIRION services accelerate the preclinical phase while developing appropriate upstream and downstream processes and analytic tools to enable a smooth Project Transfer – speeding up the project transfer and process development phase at a CDMO by up to 8 months.
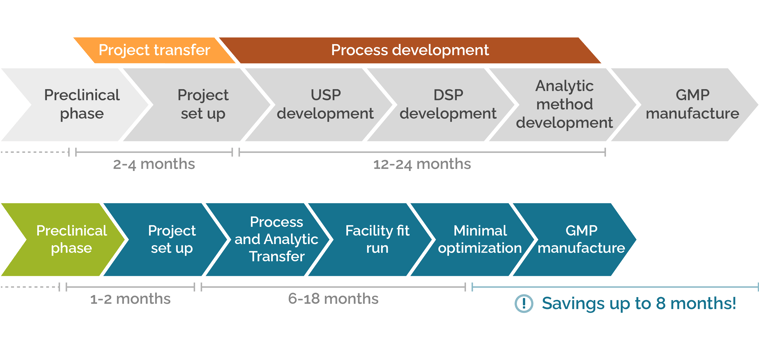
Contact SIRION today to learn how you can save up to 8 months development time for GMP manufacture..
