Lentivirus Manufacturing
Custom Lentivirus Vectors for Discovery and Preclinical Applications
Lentivirus is the tool of choice for fast, efficient, and cost-effective generation of stable homogeneous cell models for strong gene expression or gene knockdown. In gene therapy, Lentiviruses are widely used for CAR-T cell therapy applications.
SIRION Biotech offers custom lentiviral vector engineering and manufacture in scales from 3E6 IU up to 2.5E9 IU with constitutive or inducible promoters for in vitro and in vivo applications. Our lentiviral vectors are 3rd generation vectors based on 3’SIN Technology, and are pseudotyped with VSV-G protein (vesicular stomatitis virus G protein) to allow robust replication and entry into the broadest variety of cells.
Lentivirus for Cell Therapy provides more detail about the support and services we can offer gene therapy clients.
SIRION Benefits at a Glance
Our custom engineering service allows integration of virtually any desired expression construct
-
Personal consultancy and planning for each project by lentivirus experts
-
Flexible engineering service for integration of virtually every desired transgene
-
Guaranteed standards for reliable batch-to-batch consistency
-
Various scales and purities to fit any step from R&D to GMP-ready
-
Customized QC packages
-
CDMO network for smooth transfer to manufacturing under GMP license
Characteristics of Recombinant Lentivirus
Lentivirus offer the most versatile method for gene delivery. Lentivirus infects a broad panel of dividing and non-dividing cells, including primary cells, immortalized cell lines, and hematopoietic stem cells. Recombinant lentiviral vectors offer flexible vector design, and insertion of an expression cassette which may include several genes with a total capacity up to 5 kb including the promoter, gene of interest (GOI), WPRE, etc.
-
Application field from in vitro to in vivo
-
Stable gene expression due to integration of lentivirus into actively transcribed sites of the genome
-
Reliable gene delivery into dividing and non-dividing cells
-
Large packaging capacity
Lentivirus Engineering
Lentivirus are retroviruses with a single stranded RNA genome. It has three main genes coding for the viral proteins gag, pol, and env. These genes are responsible for viral capsid and matrix proteins (gag), viral integration and transcription of the carried GOI (pol), transmembrane proteins required for host cell entry (env).
Lentiviral vectors contain only the viral cis-regulatory elements necessary for genome integration, transcription, and packaging of the vector RNA. All genes coding for viral proteins are removed to provide space for insertion of the transgene DNA.
3’SIN technology for safe and effective lentiviral vectors
To ensure safety by preventing viral replication in target cells, 3rd-generation lentiviral vectors have much of the viral genome moved into separate packaging plasmids. SIRION Biotech uses 3’SIN (self-inactivating) technology – a 400 bp deletion in the U3 region of the 3‘UTR which inactivates its promoter activity. During integration of viral DNA into the host genome, this 3‘SIN LTR is transferred to the 5‘end, resulting in a provirus lacking U3 promoter activity at both ends. 3’SIN technology thus enables controlled gene expression from internal promoter(s).
In addition to the plasmid carrying the GOI, 3’SIN technology requires three packaging plasmids to provide all the protein components necessary for LV packaging and generation of infectious viral particles. Lentiviral vectors are pseudotyped with VSV-G protein for mediation of fusion of the viral envelope with a host cell.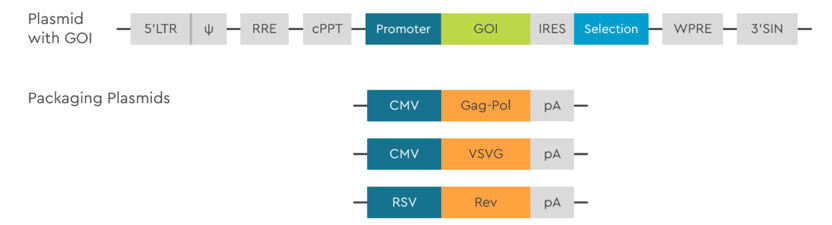
Figure 1: Schematic representation of the lentiviral vector genome organization. In the expression plasmid, viral cis-elements are removed and replaced with the gene of interest (GOI), promoter, and selection marker. To manufacture lentivirus vectors, three packaging plasmids are mixed together with the expression plasmid.
SIRION 3’SIN Lentivirus Vector Characteristics
-
No overlapping sequences between the co-transfected vectors eliminates any homologous recombination
-
VSV-G protein pseudotyping provides broad host tropism
-
Controlled gene expression from internal promoter(s)
Constitutive and inducible lentiviral vectors
SIRION Biotech offers a broad portfolio of constitutive and inducible promoters for lentiviral vectors.
Constitutive lentiviral vectors
Constitutive lentiviral vectors available from SIRION infect both dividing and non-dividing cells and offer:
-
Long-term gene overexpression and silencing
-
A variety of strong and ubiquitous promoters (CMV, Ubc, EF1α, etc.)
-
Custom promoters
-
A variety of selection markers (Puro, Neo, Blasti, etc.)
-
IRES or 2A sequences for bicistronic vectors
-
VSV-G pseudotyping for broad cell tropism

Figure 2: Schematic representation of a constitutive lentiviral vector construct for cell model generation. Promoter and resistance can be selected based on experimental needs.
Inducible TET lentiviral vectors
The Tet-system (Tetracycline-Controlled Transcriptional Activation) allows efficient, reversible, and controllable gene expression via the antibiotic doxycycline (Dox). Tet-On and Tet-Off vectors carry doxycycline-responsive transactivator protein genes along with the inducible promoter controlling gene of interest. The Tet-on system responds to Dox faster than the Tet-off system.
Tet All-in-ONE uses the latest 3G TET-On technology and provides both of these genes in a single vector. If the size of GOI exceeds the insertion capabilities for the All-in-ONE vector, the 2 vector system can still be used to deliver inducible large constructs. SIRION will work with you to determine the best system for your needs.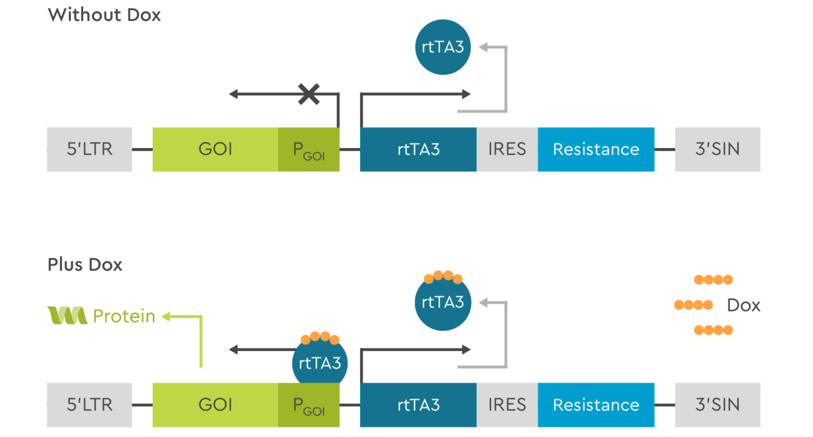
Figure 3: Schematic representation of the 3G Tet-On All-in-ONE system. In absence of Doxycycline in the medium, the transactivator protein rtTA3 is constitutively expressed but cannot bind to the promoter driving transcription of the GOI to activate it. When Doxycycline is added to the medium, it binds to rtTA3 and modifies its conformation, allowing it to bind to the promoter and activate transcription of GOI.
Advantages of Tet All-in-ONE inducible vectors:
-
300–400 times increased expression levels for induced cells
-
More than 10.000-fold induction over background
-
Improved design for high sensitivity and activity – uses 100-fold less Dox and produces 7-fold more activity than the original Tet-On
-
Up to 4 kb packaging capacity
-
No need for rounds of clonal selection
-
Suitable for primary cells
-
Can be manufactured to a high-titer
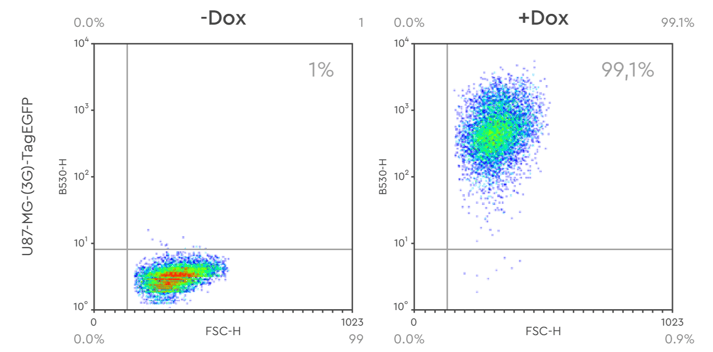
Figure 4: FACS analysis of stable U87MG cell pools with Tet-inducible expression of Tag-eGFP. Around 99% of the cells express Tag-eGFP when induced with Doxycycline.
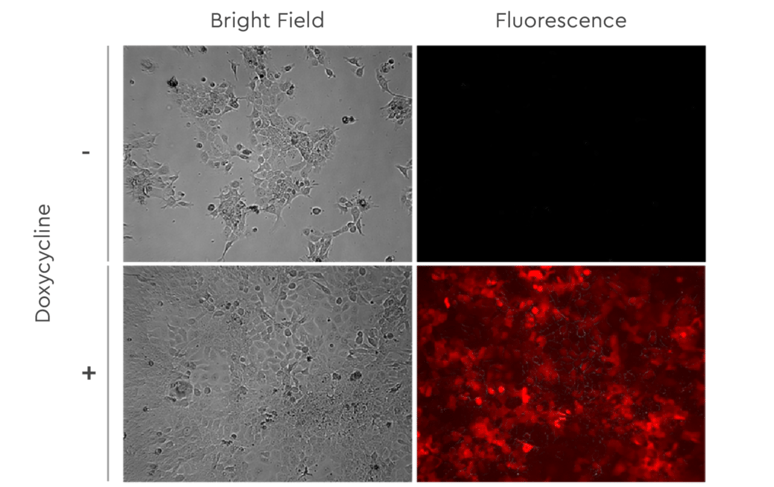
Figure 5: Fadu cells stably transduced with lentiviral vectors for inducible expression of RFP. Imaging after 48h in medium with or without 1,0 µg/ml Doxycycline.
Fields of Application for inducible vectors include:
-
Stable inducible cell model generation
-
Circumvention of cell adaption to stable genetic modification
-
Characterization of toxic gene modulations
Lentiviral Vector Manufacturing Service
SIRION works with you to provide the service you need, from direct manufacture using your existing lentiviral expression plasmid, to full service where we synthesize the GOI, and clone and manufacture the lentiviral vector particles.
Workflow using customer expression plasmid
-
Customer provides plasmid map, gene sequence of the vector
-
Feasibility check by SIRION molecular biologists, costs estimation
-
Strategy confirmation by customer
-
Timeline estimation
-
Custom expression plasmid amplification (for large scale projects), manufacture, QC.
-
Shipment of the particles and report to the customer
Workflow Using Customer Gene Sequence
Customer provides sequence of GOI or NM-number
-
Feasibility check of gene sequence by SIRION molecular biologists, preparation of the construct design drafts, vector generation strategy setup and cost estimation
-
Design and strategy confirmation by customer
-
Timeline estimation
-
cDNA synthesis, cloning, plasmid amplification, manufacture, QC.
-
Shipment of the particles and report to the customer

Upstream and Downstream Processes
The manufacturing process for lentivirus vectors includes production (cell line transfection and virus harvesting) and purification stages (centrifugation, filtration), followed by formulation, QC, and aliquoting.
Lentiviral Vector Production Process
| Co-transfection of: | 3rd generation packaging plasmid mix lentiviral expression plasmid |
| Production cell line | Adherent HEK293T Cells, cell factories (0.8 L, low-serum medium) for standard and medium scale productions. For large and XX-large scales LV-MAX™, Lentiviral Production System (ThermoFisher). |
| Transfection reagent | PEI |
| Virus harvest | 48h after transfection |
| LV particles isolation | From supernatant |
Lentiviral Vector Purification Process
| Crude Lysate preparation | Centrifugation and treatment with 30 U/ml Endonuclease PEG-precipitation of LV particles from culture medium |
| Sterile filtration | For in vivo use |
| Formulation buffer | Serum-free CD293 (Life Technologies) |
| Aliquoting | Standrad or custom |
Quality Control and Analytics
SIRION performs standard quality control procedures on every construct. We routinely determine the physical and infectious titer and offer additional analytics according to customer need for more extensive quality control of vectors (Table 4).
Table 3: Standard lentiviral vector QC methods
| STANDARD QC METHOD | DESCRIPTION |
| Physical genomic particle titer (GC/ml) | Viral RNA is lysed and prepared using the QIAamp® Viral RNA Mini Kit from QIAGEN. The genomic titers are titrated against a standard curve included in the Lenti-X™ qRT-PCR Titration Kit. |
| Functional titer (IU/ml) | Relative expression level of the vector transgene is measured by qRT-PCR relative to a control virus and a reference gene (hTOP1). |
| TU titer (TU/ml) | The calculation is based on an average GC/TU quotient of 10.2 which was determined experimentally using the LentiX provirus kit (from Clontech) from > 200 individual determinations. |
Table 4: Optional analytical QC methods for lentiviral vectors
| OPTIONAL QC METHOD | DESCRIPTION |
| Endotoxin test | Endosafe-nexgen-PTS Spectrometer |
| Functional testing in HEK293 cells | Antibiotic selection, fluorescence detection, luciferase measurement |
| Functional testing in custom cells | Antibiotic selection, fluorescence detection, luciferase measurement |
| Sterility test | Incubation in CASO-Bouillon (Heipha) |
| Mycoplasma test | PCR-based assay |
| TU titer (TU/ml) | Determined experimentally using the LentiX provirus kit (from Clontech) |
Scales and Timelines
SIRION offers several production stages and quantities for all levels of preclinical applications. Production parameters can be adapted to specific projects after further consultation with the SIRION team.
| CUSTOM GENE SYNTHESIS |
|
| cDNA synthesis | up to 21 business days Performed by GeneArt |
| LV CUSTOM VECTOR CLONING |
|
| Fast track (Gateway based) Classical cloning (Gibson assembly based) |
2-3 weeks |
| LV «CONSTITUTIVE» PRODUCTION |
||
| SCALE | SERVICE TYPE | TIMELINES |
| >3E6-1E7 IU | STANDARD | 3-4 weeks |
| >3E7-1E8 IU | MEDIUM | 3-4 weeks |
| Lentiviral vector productivity evaluation | 3-4 weeks | |
| >5E8 IU | LARGE | 5-6 weeks |
| >1E9 IU | X-LARGE | 6-7 weeks |
| >2.5E9 IU | XX-LARGE | 6-8 weeks |
| LV «INDUCIBLE» PRODUCTION |
||
| SCALE | SERVICE TYPE | TIMELINES |
| >3E6-1E7 IU | STANDARD | 3-4 weeks |
| >3E7-1E8 IU | MEDIUM | 3-4 weeks |
| Lentiviral vector productivity evaluation | 3-4 weeks | |
| >5E8 IU | LARGE | 5-6 weeks |
| >1E9 IU | X-LARGE | TBD |
LentiBOOST Transduction Enhancer
LentiBOOST is our proprietary transduction enhancer that improves lentiviral transduction efficiency and VCN across a wide range of clinically relevant cell types. For more information please visit our LentiBOOST page here.
