AAV Manufacturing
Custom AAV Vectors for Discovery and Preclinical Applications
Custom AAV Vectors for Discovery and Preclinical Applications
Custom AAV Vectors for Discovery and Preclinical Applications
SIRION Biotech AAV manufacturing and engineering services are renowned for providing the highest quality products.
Our sophisticated technology platform allows the integration of virtually any desired expression construct within the vector’s cloning capacity. Batch sizes range from 1E12 VG (vector genomes) for small explorative experiments all the way to 1E15 VG to satisfy complete experimental cohorts.
SIRION Biotech manufactures a range of AAV serotypes in combination with tissue specific promotors to further increase gene expression precision. Additional in-house features of the SIRION BIOTECH adeno-associated virus technology platform include surface modified particles for cell-specific transduction.
AAV for Gene Therapy provides more detail about the support and services we can offer gene therapy clients.
SIRION’s Benefits at a Glance
-
Personalised consultation and planning of your custom project by AAV experts
-
ISO standards for reliable batch-to-batch quality and consistency
-
Various scales and purities to fit projects from R&D and preclinical to NHP studies
-
Wide selection of expression vectors and serotypes
-
QC packages ranging from basic to characterization in accordance with regulatory guidelines
-
Premade full and empty capsids as controls for analytics or process development
-
CDMO network for smooth transfer to manufacturing under GMP licence
Recombinant AAV Vectors
Recombinant AAV (rAAV) vectors are developed by excision of the rep and cap genes and insertion of an expression cassette of the gene of interest (GOI) flanked by the two ITRs (Figure 1). The rep/cap genes and the helper virus-derived components are provided in separate plasmid(s). A GOI up to 4.5 kb can be inserted into single strand rAAVs. For self-complimentary rAAV vectors (scAAVs), the GOI size is limited to 2.5 kb.
Schematic representation of the AAV2 genome organization
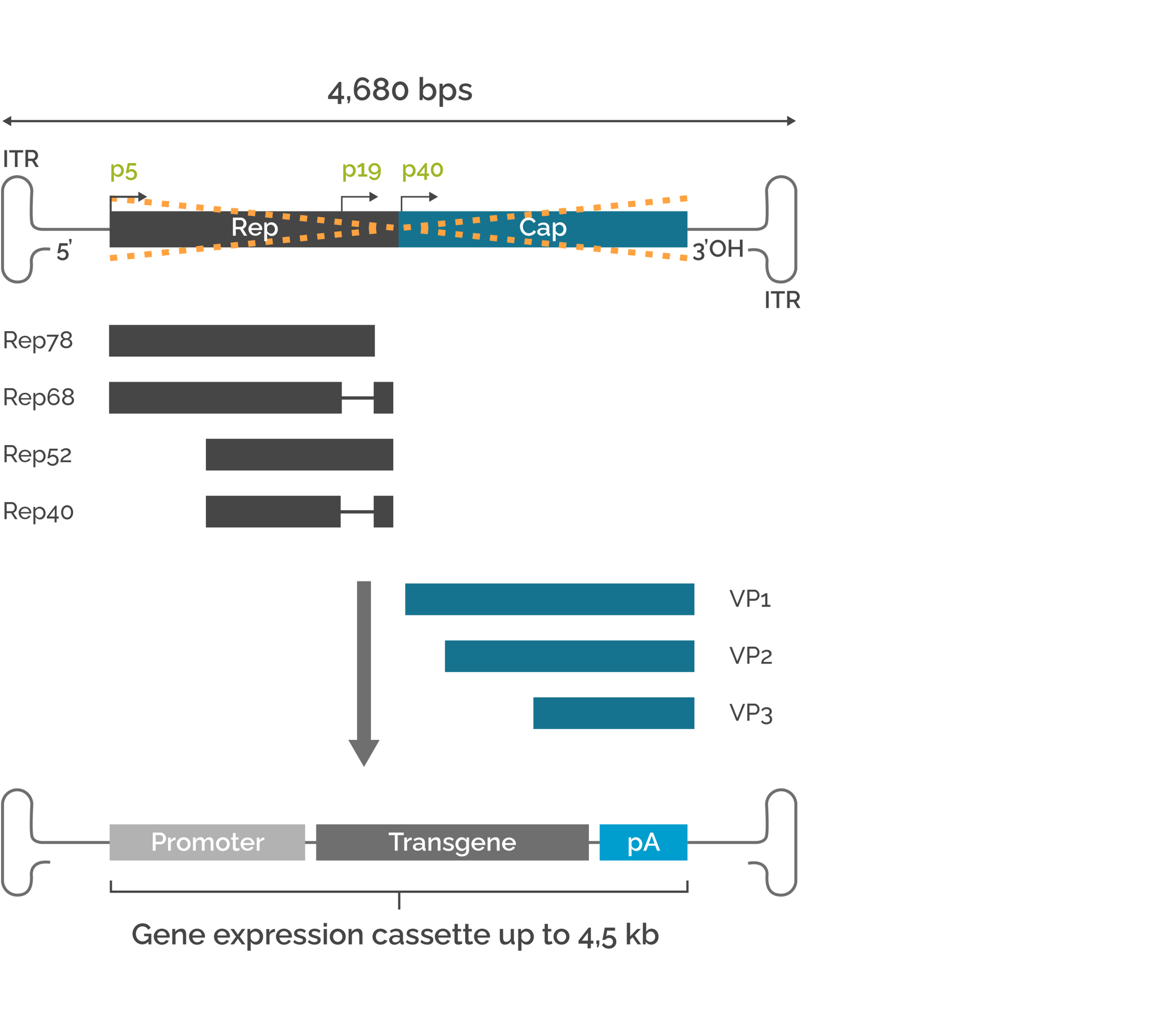
Figure 1. Schematic representation of the AAV2 genome. The rep and cap genes encode proteins required for AAV2 life cycle (Rep) and capsid proteins (Cap). To generate a rAAV vector, rep and cap genes are removed and a gene of interest up to 4.5 kb can be inserted.
AAV Serotype Recommendations
Several naturally occurring AAV capsid serotypes are available, all exhibiting varying tissue- and cell tropisms. Specific serotypes are recommended to target specific tissues in your experiments. For example, AAV6 efficiently transduces cardiac tissue (Figure 2).
In addition to naturally occurring serotypes, a growing array of artificially engineered variants continue to emerge from the gene therapy field. SIRION is highly experienced and active in handling both standard serotypes and novel capsid variants.
Table 1 provides a general overview of AAV serotype specificity based on literature and SIRION’s experience, as a starting point for serotype selection. Serotype tropisms in the CNS are reviewed in more detail in Table 2.
Further we can support the selection of serotypes for specific cell types.
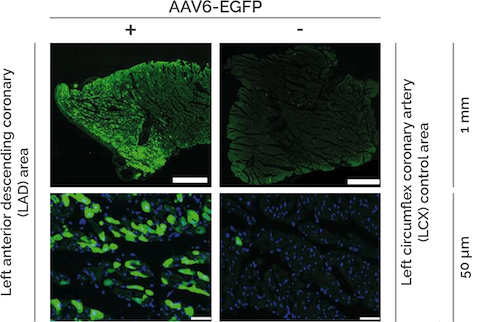
Figure 2. Recombinant AAV6-GFP injection in test animals. 10 weeks after injection. Data provided by PD Dr. Oliver J. Mueller, Otto-Meyerhof-Zentrum University Clinic Heidelberg.
| TARGET TISSUE | AAV Serotype |
| Skeletal muscle | AAV1***, AAV6**, AAV7*, AAV8***, AAV9*** |
| Heart | AAV1***, AAV3*, AAV4*, AAV6**, AAV8*, AAV9** |
| Lung | AAV1*, AAV4**, AAV5**, AAV6**, AAV9*** |
| Liver | AAV1*, AAV3***, AAV4*, AAV5**, AAV6*, AAV8** |
| T Cells | AAV6* |
| CNS | AAV1*, AAV2**, AAV5**, AAV6*, AAV7*, AAV8**, AAV9** |
| Hematopoietic stem cells | AAV2*, AAV3*, AAV6* |
| Embryonic stem cells, iPSC | AAV6* |
| Kidney | AAV2*, AAV4**, AAV5* |
| Pancreas | AAV1, AAV6* |
Color Key: * mice / * large animals: dogs, pigs, sheep, rabbits, cats / * human (tissue or clinical trial) / AAV serotypes specificity verified by SIRION
Table 1. Overview of AAV serotype tropism as a basis for AAV serotype selection (Srivastava, 2016, Vance et al., 2015, Keeler & Flotte, 2019, Wang et al., 2019, Li & Samulski, 2020).
| CELL TYPE | AAV SEROTPE |
| Striatal neurons | AAV1/2 |
| Hypothalamus | AAV1/2, AAV5 |
| RPE/Ganglion cells | AAV2, AAV4, AAV5 |
| Photoreceptors, Astrocytes | AAV5 |
| Motoneurons | AAV6 |
Table 2. AAV serotype recommendation for CNS/ Eye Targeting. Recommendations based on experimental data (Trapani et al, 2008, Davidson et al, 2018).
Promoter Selection
Promoter modifications can be powerful tools to enable cell type specific transgene expression. For example, rAAV5 transduces both rod and cone cells in the retina, but using cell-type specific promoters allows for targeted expression in either cell type. (Figure 3). SIRION offers a panel of in vivo validated tissue-specific promoters and can support expression cassette development.
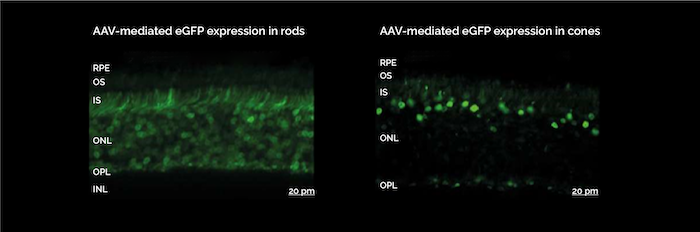
Figure 3. Recombinant AAV5 vectors enable targeted expression in rod or cone cells using different promoters: two-week-old C57-B6/N WT mice received a dose of 1E9 GC AAV5 expressing eGFP under control of a mouse rhodopsin promoter (A) or s-opsin (B) promoter. 4 weeks after treatment. Data provided by Prof. Dr. Martin Biel, PD Dr. Stylianos Michalakis, Ludwig Maximilians University, Munich.
Recombinant AAV Manufacturing Service
SIRION offers a modular service approach for generation of rAAV vectors. Customers may either choose SIRION’s AAV2-based backbone or provide their own expression plasmid.
A complete/all-inclusive project begins with sourcing cDNA templates containing all custom elements followed by cloning of these elements into an AAV expression plasmid, plasmid amplification, particle production and QC.
Figure 4. The general overview of SIRION’s rAAV vector generation
Raw Material
Choose SIRION’s AAV2-based backbone or supply an expression plasmid*. We will check feasibility in silico prior to production.
* SIRION can only manufacture IP-protected serotypes under the client’s license umbrella.
Vector Design
-
in silico vector design and engineering.
-
Fast Track (Gateway-based) or classical cloning (Gibson)
-
Vectors optimized for clinical application
SIRION has extensive experience designing clinically-compliant vectors with optimized ‘therapeutic payload’ cassettes and transfer vector backbones, to reduce toxicity and improve efficacy. You can find more information about our AAV Vectors for Cell and Gene Therapy here.
Production and Purification
SIRION’s customizable modular approach allows us to determine the most cost-effective upstream (USP) and downstream (DSP)processes to meet client-specified quality attributes, e.g., cross-packaging of non-AAV cassette material and empty/full capsid ratios.
SIRION offers production of preclinical batches of up to 50 L.
For the downstream process, SIRION offers 1 step (iodixanol gradient) or 2 step (affinity capture, followed by iodixanol gradient) purification. While 1-step purification provides excellent results, we recommend 2-step purification for late stage clinical applications.
Figure 5: Modular system for design and production of AAV vectors
QC and Analytics
SIRION offers basic and standard packages. Additional QC assays can be ordered separately.
| QC PACKAGE | DESCRIPTION |
| BASIC | Genome titration |
| STANDARD | Genome titration, Purity check on silver gel, Integrity check, Endotoxin test |
| STANDARD analytic | Capsid titer, Purity check on Silver gel, Endotoxin test |
Selection of Available QC Assays
Please inquire for additional assays to analyse identity, potency, purity, and safety of the AAV vectors.
Recommendations for Purification and QC Selection
| PROPOSED EXPERIMENT | RECOMMENDATION | |
| Purification | QC Package | |
| In vitro transduction of cells & PoC in vivo experiments for which purity is not a crucial factor | 1-STEP | BASIC / STANDARD |
| Systemic delivery for targeting organs (liver, lung, muscle, CNS) in small animals at early stage preclinical application OR in large animals (non-human primates) at late stage of preclinical application | 2-Steps | PREMIUM / PREMIUM PLUS |
| Direct injections into tissue (eyes and brain) late stage of preclinical applications | 2-Steps | PREMIUM PLUS |
Premade AAV Capsids with GFP
Our high-quality premade AAV particles are ideal for pilot studies, or as standardized test material for product, process, or assay development.
Full AAV Premade Capsids
-
GFP overexpressing AAV vectors
-
2- step purification via FPLC and Iodixanol gradient UC
-
Premium QC
-
25 μl @ 1.0E13 VG (vector genomes)/mL
-
in vivo buffer (PBS+0,001% Pluronic)
Premade AAV particles are available for all common serotypes.
Empty AAV Capsids
Purified empty AAV capsids have tremendous value for several stages in the gene and cell therapy-development journey.
SIRION Offers:
- 100% empty capsids with SIRION's innovative in-house production platform
- De novo manufacture of up to 5E15VP (virus particles)
- Cost-efficient and well characterized test and control material
Empty AAV Capsids are available for all common serotypes in 2 production scales:
- Standard Scale -- 100μl @ 1.0E13 VP (virus particles)/ml
- Large Scale – 1 ml @ 1.0E13 VP (virus particles)/ml
Each production run includes:
- AAV empty particles of the requested serotype;
- 1-step purification
- Premium-analytic QC in vivo buffer (PBS+0,014% Tween 20)
Commercial Product, Process, and Assay Development
Well characterized AAV empty capsids are highly suitable as standardized reference materials for commercial product-, process- and assay development. Using SIRION’s highly purified empty capsids ensures correct quantitation and determination of detection limits.
In-house Testing and Control Material
Companies developing gene therapies use purified AAV empty capsids as in-house test and control material for accurate interpretation of assays, reliable titration of AAV vector lots, or to assess the effects of the therapeutic AAV vector compared to an empty control capsid.
Immunogenicity and Seroprevalence Testing
Wild-type AAV virus is highly prevalent in our society with up to 40% of some populations carrying existing neutralizing antibody titres against AAV serotypes. A potential immunogenic reaction to the AAV-based drug product impairs the therapeutic outcome of gene therapies. To reduce negative side effects, it is crucial to measure pre-existing anti-AAV or neutralizing antibody titers prior to accepting prospective subjects into clinical trials.
AAV empty capsids are a cost effective and reliable solution for immunogenicity or seroprevalence testing of specific AAV serotypes.
Services and Timelines
SIRION offers several production stages and quantities for all levels of preclinical application. Production parameters can be adapted to specific projects after further consultation with the SIRION team.
| cDNA SYNTHESIS | TIMELINE |
| cDNA synthesis of transgene to fit for pENTRY plasmid* *performed by third party supplier |
ca. 18 days |
| rAAV CUSTOM VECTOR CLONING | TIMELINE |
| Fast-Track Cloning Cloning into an AAV2-based expression vector based on Gateway® technology |
1-2 weeks |
| Classical Cloning Cloning into an AAV2-based expression vector using PCR-based cloning |
3-4 weeks |
rAAV R&D Timelines
| SCALE | SERVICE | PURIFICATION | CONCENTRATION | BUFFER | PLASMID** | TIMELINES |
| 1E12 VG | PILOT | 1-STEP | Not specified | 40 % iodixanol | 0.2 mg | 2-3 weeks |
| 1E13 VG | PILOT+ | 1-STEP | Not specified | 40 % iodixanol | 1 mg | 2-3 weeks |
| 1E13 VG | STANDARD | 1-STEP | 1E13 VG/ML* | Choice of in vivo buffers | 1 mg | 2-3 weeks |
| 1E13 VG | PREMIUM | 2-STEP | 1E13 VG/ML* | Choice of in vivo buffers | 2 mg | 3-4 weeks |
*For rAAV1, rAAV2 final concentration up to 5E12 VG/ml. **For exact amounts of raw materials needed (rAAV expression plasmid) – please inquire directly.
Table 8. Recombinant AAV R&D production timelines.
To assess productivity, production in 1 x CF10 prior to large-scale manufacture (>1E13 VG) will be performed to guarantee successful process scale-up. Production timelines depend on the results of the productivity assessment.
rAAV LATE PRECLINICAL
| SCALE | PURIFICATION | CONCENTRATION | BUFFER | TIMELINES |
| 5E13 VG | 2-STEP | 1E13 VG/ML* | In vivo | TBD |
| 1E14 VG | 2-STEP | 1E13 VG/ML* | In vivo | TBD |
| 2E14 VG | 2-STEP | 1E13 VG/ML* | In vivo | TBD |
| 5E14 VG | 2-STEP | 1E13 VG/ML* | In vivo | TBD |
*For AAV1, AAV2 final concentration up to 5E12 VG/ml.
rAAV ANALYTICS
| SCALE | PURIFICATION | BUFFER | TIMELINES |
| 5E13 PP* | 1-STEP | In vivo | 3-4 weeks |
| 1E14 PP | 1-STEP | In vivo | 4-6 weeks |
| 2E14 PP | 2-STEP | In vivo | 5-7 weeks |
*PP refers to physical particles
Project Process Example
SIRION offers flexible and customizable projects, supported by expert AAV specialists from start to finish.
-
Check of feasibility and vector design: each project starts with detailed planning together with our highly skilled AAV specialists to ensure the best possible outcome.
-
Plasmid cloning options: maximal capacity is 4.5 kb for ssAAV and 2.5 kb for scAAV. Both 2 and 3 plasmid system packaging are available.
-
Expression plasmid can be provided by SIRION or by customer (including the proof by specific enzyme digestion of both left and right ITRs integrity and plasmid maps).
-
Packaging and helper plasmids can be provided by SIRION or by customer.
-
-
Serotype selection: AAV serotype 1 – 6, 8, 9, rh10, DJ, etc.-
-
Promoter selection:
-
Ubiquitous and strong promoters (CMV, EF1a, Ubic)
-
Cell type specific promoters (CNS, heart, photoreceptors, liver, immune cells).
-
Tet-inducible promoters (License is required for customer applications).
-
-
Reporter gene co-expression selection (GFP, YFP, RFP, LUC, etc.)
-
Scale (titer) identification: from small-scale up to large preclinical scales (1E15VG)
-
Concentration
-
Purification: all serotypes can be purified in 1- or 2-steps using affinity chromatography and\or iodoxinal gradient ultracentrifugation.
-
A selection of QC packages suitable for basic research through to late preclinical studies
-
Formulation for in vitro or in vivo applications
-
Aliquoting: standard or custom.
